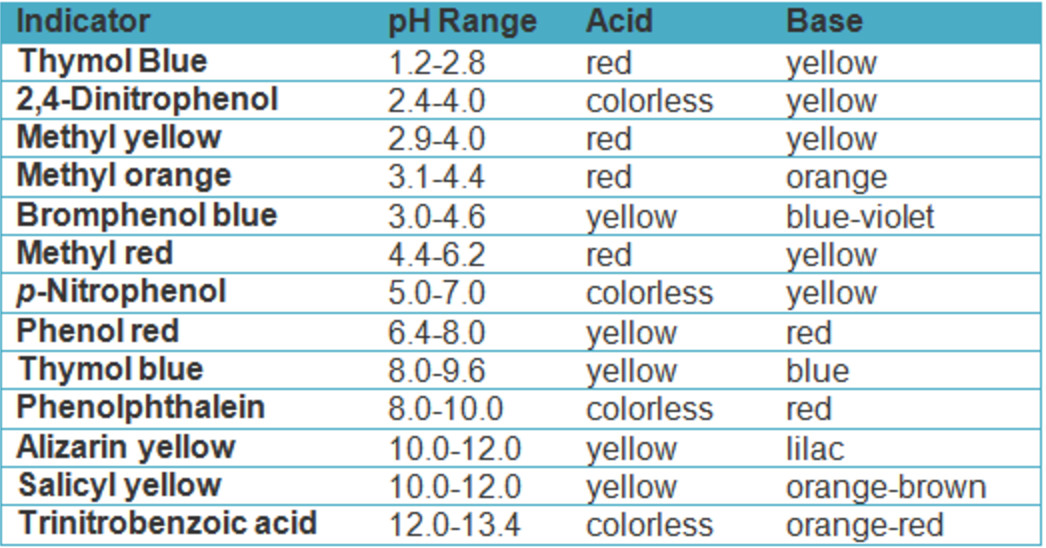
Indicator Titration Equation . a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The graph shows the results obtained. The best selection would be an indicator that has a color. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. This titration is used to find out the unknown concentration of an acid or base through neutralizing with an acid or base of known concentration. This page assumes that you know.
from exogrvkja.blob.core.windows.net
titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. This page assumes that you know. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The best selection would be an indicator that has a color. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. This titration is used to find out the unknown concentration of an acid or base through neutralizing with an acid or base of known concentration. The graph shows the results obtained. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base.
Indicators For Titrations at Leslie Jackson blog
Indicator Titration Equation a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The best selection would be an indicator that has a color. This page assumes that you know. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. The graph shows the results obtained. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. This titration is used to find out the unknown concentration of an acid or base through neutralizing with an acid or base of known concentration.
From byjus.com
in the titration of Na2CO3 by HCl using methyl orange indicator, thevolume required at the Indicator Titration Equation titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. The best selection would be an indicator that has a color. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. This page assumes that you know. The graph shows. Indicator Titration Equation.
From theedge.com.hk
Chemistry How To Titration The Edge Indicator Titration Equation The best selection would be an indicator that has a color. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. This titration is used to find out the unknown concentration of an acid or base through neutralizing with an acid or base of known concentration. a titration is a volumetric. Indicator Titration Equation.
From pressbooks.bccampus.ca
8.7 AcidBase Titrations Chemistry for Chemical Engineers Indicator Titration Equation The best selection would be an indicator that has a color. This page assumes that you know. The graph shows the results obtained. titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid. Indicator Titration Equation.
From www.slideserve.com
PPT pH of weak electrolites Indicators The HendersonHasselbalch equation Titration PowerPoint Indicator Titration Equation a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. The best selection would be an indicator that has a color. the above equation. Indicator Titration Equation.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Indicator Titration Equation the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. This page assumes that you know. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. titration curves help us pick an indicator that will provide a sharp color change. Indicator Titration Equation.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Indicator Titration Equation the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. This page assumes that you know. This titration is used to find out the unknown concentration of an acid or base through neutralizing. Indicator Titration Equation.
From www.studocu.com
Experiment 3 lectures DoubleIndicator Titration Method ACIDBASE TITRATION volumetric Indicator Titration Equation The best selection would be an indicator that has a color. titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. This titration is used to find out the unknown concentration of an acid or base through neutralizing with an acid or base of known concentration. the above equation works. Indicator Titration Equation.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Indicator Titration Equation titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. This page assumes that you know. The best selection would be an indicator that has. Indicator Titration Equation.
From www.youtube.com
U11L6 Indicators, Neutralization Reaction, and Titrations Calculations YouTube Indicator Titration Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The graph shows the results obtained. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. titration curves help us pick an. Indicator Titration Equation.
From baharlabware.com
تیتراسیون و انواع آن تیتراسیون چیست محلول تیتراسیون بهار تجهیز Indicator Titration Equation the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. This page assumes that you know. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. The best selection would be an indicator that has a color. a titration is. Indicator Titration Equation.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators of Acid Base Titration Indicator Titration Equation titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. the titration curves shown in figure 14.20 illustrate the choice of a suitable indicator for specific titrations. The graph shows the results obtained. a titration is a volumetric technique in which a solution of one reactant (the titrant) is. Indicator Titration Equation.
From cedpcfbl.blob.core.windows.net
Titration Indicator Analysis at Donna Kendall blog Indicator Titration Equation This titration is used to find out the unknown concentration of an acid or base through neutralizing with an acid or base of known concentration. The best selection would be an indicator that has a color. The graph shows the results obtained. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid. Indicator Titration Equation.
From www.numerade.com
SOLVED 3. What is an indicator and how does it function in a titration experiment? 4 By using Indicator Titration Equation This page assumes that you know. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. This titration is used to find out the unknown concentration of an acid or base through neutralizing with an acid or base of known concentration. titration curves. Indicator Titration Equation.
From cesttiwp.blob.core.windows.net
Titration Volume Formula at Charles Pope blog Indicator Titration Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the. This page assumes that you know. the titration curves shown in figure. Indicator Titration Equation.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive Indicator Titration Equation titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. This titration is used to find out the unknown concentration of an acid or base through neutralizing with an acid or base of known concentration. This page assumes that you know. the above equation works only for neutralizations in which. Indicator Titration Equation.
From www.slideserve.com
PPT pH of weak electrolites Indicators The HendersonHasselbalch equation Titration PowerPoint Indicator Titration Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. The graph shows the results obtained. the titration curves shown in figure 14.20 illustrate the choice of a suitable. Indicator Titration Equation.
From www.slideshare.net
Acid base titration Indicator Titration Equation the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. This titration is used to find out the unknown concentration of an acid or base through neutralizing with an acid. Indicator Titration Equation.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators of Acid Base Titration Indicator Titration Equation The graph shows the results obtained. The best selection would be an indicator that has a color. titration curves help us pick an indicator that will provide a sharp color change at the equivalence point. a titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second. Indicator Titration Equation.